Details of the Drug
General Information of Drug (ID: DMIYJFV)
| Drug Name |
Nicorandil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adancor; Dancor; Ikorel; Nicorandilum; Sigmart; Aventis Brand of Nicorandil; Aventis Pharma Brand of Nicorandil; Merck Brand of Nicorandil; Merck Lipha Sante Brand of Nicorandil; Nicorandil Aventis Brand; Nicorandil Merck Brand; Rhone Poulenc Rorer Brand of Nicorandil; SG 75; SG75; Ikorel (TN); Nicorandilum [INN-Latin]; RP-46417; Rhone-Poulenc Rorer Brand of Nicorandil; SG-75; Sigmart (TN); Nitrate, 2-Nicotinamidethyl; Nitrate, 2-Nicotinamidoethyl; N-(2-Hydroxyethyl)nicotinamide nitrate; Nicorandil (JP15/USAN/INN); Nicorandil [USAN:BAN:INN:JAN]; N-(2-Hydroxyethyl)nicotinamide nitrate (ester); N-[2-(Nitrooxy)ethyl]pyridine-3-carboxamide; 2 Nicotinamidethyl Nitrate; 2 Nicotinamidoethyl Nitrate; 2-(Nicotinamido)ethyl nitrat; 2-(Pyridine-3-carboxamido)ethyl Nitrate; 2-(pyridine-3-carbonylamino)ethyl nitrate; 2-Nicotinamidethyl Nitrate; 2-Nicotinamidoethyl nitrate; 2-[(pyridin-3-ylcarbonyl)amino]ethylnitrat
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Vasodilator Agents
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
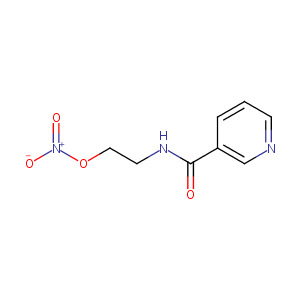 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 211.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Angina pectoris | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA40 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
